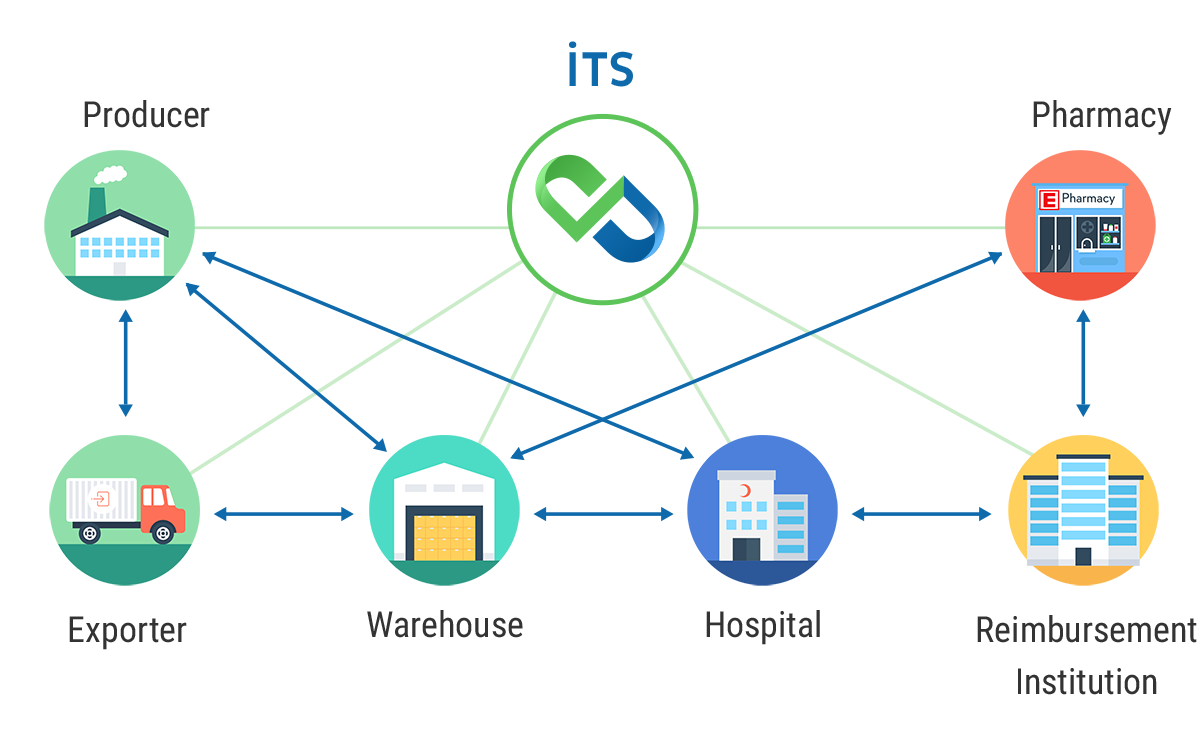
The actions that stakeholders can make according to the information given in the diagram; purchase of drugs, sales of drugs, return of the drugs, declaration of consumption (for consumption centers), verification.
Production Notification

Manufacturer/Importer Sale Notification

Import Notification

Wholesaler Sale Notification

Confirmation
This process shows if a product can or cannot be sold. If a product is shown available in a different pharmacy there will be an investigation for the real place of the said product.
Product's registrations are kept with the sale notifications. With this database, inconsistencies with the registrations can be found. The nonconforming product is deemed to be counterfeit and the unit for which the inconsistency arises is held accountable for fraud.

Deactivation
Purchase Return Notification

Purchase Confirmation
If the product is delivered without problems, it is reported to the system. In system design, purchase confirmation use is optional.
In pharmacies, the purchase confirmation notification is received when the sales transaction takes place. Because of this, it is not necessary to approve the purchase of the products given in case of emergency, but for the sale of the remaining products that cannot be done immediately, it is necessary to notify the purchase of the products concerned.



