TheFirstandOnlyintheWorld!
End-to-End Track & Trace of Pharmaceuticals

50.000+ STAKEHOLDERS
Pharmaceutical Track & Trace System is used by over 50.000 stakeholders in Türkiye.
Pharmaceuticals
Pharmacies
Manufacturers
Wholesalers
Consumption Centers
Exporters
Reimbursement Institutions
EFFECTIVE RECALL
Drug recall process ensures the shipment for a medicine’s annihilation when a negative or unexpected side effect of the medicine or specific party of the medicine is detected. Preventing the resale of recalled medicines by tracking them and returning these medicines to their producers or importers as soon as possible are vital.
STOCK MANAGEMENT &
EXPIRATION DATE CONTROL
Scanning with 2D Data Matrix eliminates the expiry date entry errors made by humans. The Pharmaceutical Track & Trace System allows stakeholders to check the inventory and expiry date information which is automatically processed into the system, leading to the better management of the products.


DATA MANAGEMENT & RATIONAL MEDICINE USE
The Pharmaceutical Track & Trace System provides a big database. Through the database, inventory levels can be tracked and traced and disease maps can be created with the analyses which specify medicines’ usage rates.
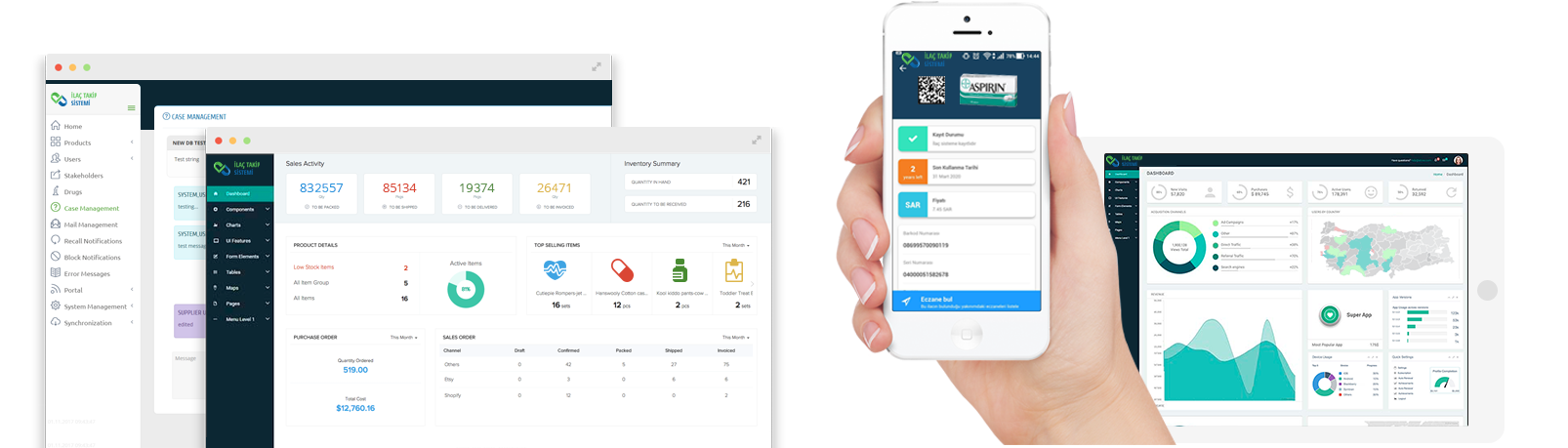
USER FRIENDLY INTERFACE
Pharmaceutical Track & Trace System, which has been developed in accordance with international standards, is designed to be suitable for heavy duty, scalable, high-performance and easy to use.

NEWS
- 21 February 2025
The World Health Organization (WHO) recently published a comprehensive […]
- 17 September 2024
Pharmaceutical Track and Trace System makes a big contribution to Türkiye’s e-Government [...]
- 24 July 2024
İTS Mobile App is a comprehensive mobile solution that enhances drug safety, accessibility and adherence for patients. But who constitutes the stakeholders and […]
- 21 December 2023
In an era where the authenticity of pharmaceuticals is a matter of life and death, the need for innovative solutions to combat the scourge of counterfeit drugs has […]










