Organizations and Institutitons
World Health Organization (WHO)
World Health Organization was established in 7th April 1948, and aimed to reach the possible health level for all people on earth. WHO has many tasks, and among all tasks the most imported one is to determine the related norms and standards for people’s health and provide the application of those norms and standards.
The anniversary of World Health Organization's establisment is celebrated in whole world as World Health Day.
GS1 – Global Standards 1
GS1, is a non-profit organization which provides global standards for labor (business) communication and sustainability. GS1 has over 1 million members and over 100 country offices.
GS1 standards is prepared to increase traceability and supply chain events. Those standards are composed a business language that shares important information about products, identifies products, locations, assets and more.
Among all know GS1 standards the most known is barcode which can electronically scanned, printed on products and contain unique information of products.
Specifications
Rational Medicine Use
Rational Medicine Use is a use of the right medicine in right time with right diagnosis, proper dosage and time period given by the doctor to a specific patient. A proper medicine should be sovereign for user and also acceptable quality and safety level. At the same time, rational medicine use is use and marketing of the medicines which are suitable for national regulation and applications, approved by authorities in terms of safety, quality and events. *
Pharmacovigilance (FV)
Scientifics works related to determine, evaluate, identify and prevent adverse effects and other possible problems related with human medicinal products.
Original and Generic Medicines
Medicines which are, unique and based on a patent molecule, proven that they have positive effect on a specific disease as a result of long term researches and clinical works.
Generic medicines are made of previously produced and launched medicines to the market. Generic medicines contain same active ingredient with same amount as original does. They are same in pharmaceutic shape and in formula. It should be proven that generics are bioequivalent with original medicines. Generics are low-cost products.
Stakeholders
Stakeholders are people, institutions and organizations which may affected from functions of the system, applied politics and results or may affect the results. Stakeholders are; producer, importer, exporter, pharmacy warehouses, pharmacies, consumption centers (hospitals, polyclinics, family doctors etc.) and consumers that are defined in Pharmaceutical Track&Trace System.
Consumables
Mostly, the medicines are given to patients one by one rather than as boxes at consumption centers such as hospitals and family doctors. For example; capsules used for an injection are chosen from a box of capsules and other capsules in the box will be used in other patient’s treatments. As seen in the example, medicines which are served one by one defined as Medical Consumables.
Recall Process
Defined as if the medicine is suspected of being defective or defective then responsible company pulls product from the market according to the determined levels. Recall process have levels according to life threatening conditions of a product.
Hospital Information Management System – HIMS
Hospital Information Management System is the system that tracks all operations of hospitals. Investigations, laboratory and pathology results, all process in operating room, hospital pharmacy operations and human resources processes are included to those operations. Hospital Information Management System is a unique system that are made of combination of software in different professions.
Global Location Number – GLN
GLN is a unique number used to identify any part of the supply chain, given by GS1. The GLN can be used to describe the physical location of medicines and legal entities.
Serial Shipping Container Code – SSCC
The SSCC is a code used by companies to specify logistics units packaged for storage or transfer in the form of any combination of commercial products. The SSCC plays an important role in ensuring traceability as it completes each logistics unit and its content differs from each other. Companies can link products to that shipping process by sharing SSCC data about the status of all units in the transfer phase via EDI or EPCIS.
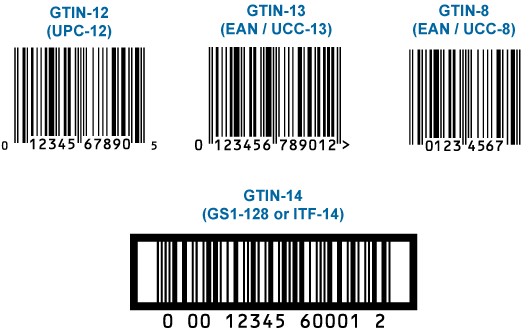
Global Trade Item Number –GTIN
GTIN is an abbreviation of the global commercial product number and is used for all levels of packaging that can be purchased and maintained. GTIN is a number created in accordance with the GTIN management standard, used to track and identify the sale, receipt and/or storage of a product.
GTIN works as a key and provides access to product information. It is used in automatic data acquisition (ADC) applications. GTIN can be 8, 12, 13, and 14 digits. 12-digit numbers North America, 8- and 13-digit numbers are used outside North America.
The 14-digit number is a 14-digit number that identifies commercial products in different packing levels, according to GS1 standards. In Turkey, 13-digit barcode is used and consists of company’s product number with prefix.
Barcode – 2D Data Matrix
The data matrix is the name given to 2D barcodes consisting of black and white cells. Numerical or textual information can be found in the data matrix. The length of the encoded data depends on the number of cells in the matrix.

The reason why data matrix is preferred in medicine boxes is that they can be read with barcode reader and products can be followed up quickly. The Pharmaceutical Track&Trace System works with the data matrix using the unique information defined for each medicine box. The data matrix contains the product's GTIN (Global Trade Item Number), serial number, expiration date and lot number.
Batch Number
It is the registration number used to distinguish a party in production from other parties.
Serial Number – SN
It is the identification number given to each of the products. The serial number used for a product can not be used again in the similar product.
Expiration Date
Specifies the last date the product can be used safely. The form of data is YYMMDD and is 6 characters long.Extensible Markup Language - XML
XML is a markup language designed to describe and standardize the data communication between data exchange systems. In use of, transferring databases, transmission of financial data, creation of file systems, and storage of scientific content. **
*Principles for the Rational Use of medicines
** https://tr.wikipedia.org/wiki/XML