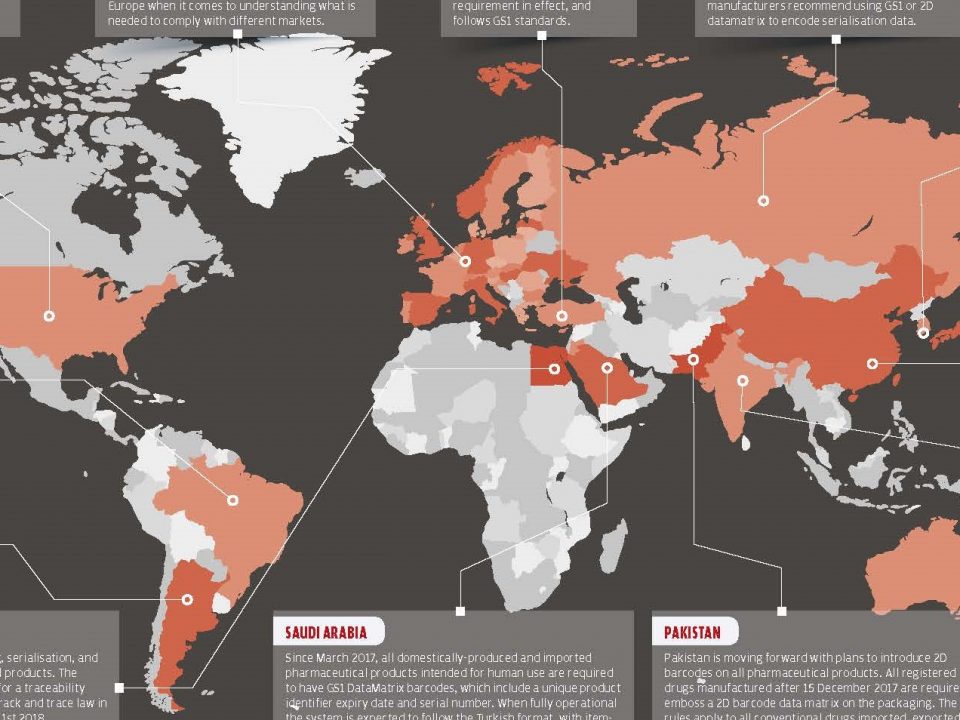
Effective Pharmaceutical Recalls
8 December 2017
Will Turkey Use the Digital Pill in Pharmaceutical Market?
22 December 2017Adverse effect; is a harmful and/or unintended effect that occurs when the medicinal product is used at normal doses for the user for the purpose of identifying, ameliorating, correcting or altering a physiological function. Pharmacovigilance covers all of the scientific work done to find, evaluate, and prevent reappearing these adverse effects that develop due to the use of medicines.
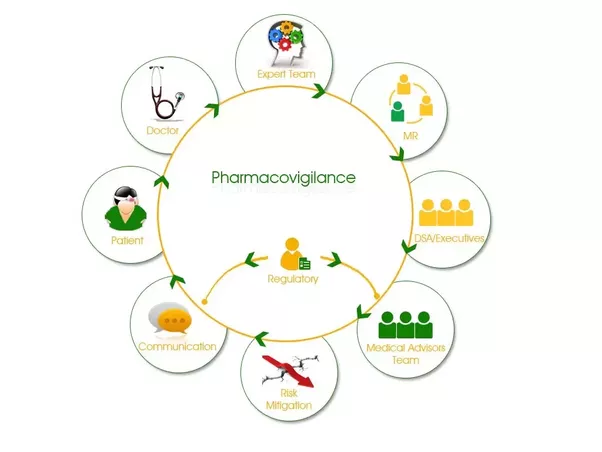
In order to ensure drug safety, it is necessary to systematically investigate adverse reactions and record information on this subject. Necessary precautions should be taken to minimize the adverse effects of drug use.
Pharmacovigilance activities to be carried out for this purpose can be exemplified as follows:
- After following the adverse reactions, sending reports to the World Health Organization (WHO) Drug Monitoring Center
- Periodically implementing risk management plans and risk assessments
- Following the health authorities’ official communication channels and the warnings about drug safety
- Regulation of training programs on pharmacovigilance and promotion of participation to said training programs